| HIRANUMA APPLICATION DATA | Automatic Titrator | Data No. | B2 | Sep. 12,2018 |
| Drugs and Medicines | Determination of sodium acetate with perchloric acid titration |
1. Abstract
Sodium acetate is widely used in chemical and medical industry (E.g. Buffer, Antifreezing agent, Alkalizing supplements, Dye chemical, Hydragogue). Determination method of sodium acetate is described on Japanese Pharmacopoeia and Japanese Industrial Standard (JIS K8371). Both methods employ perchloric acid titration method, dissolve the sample in glacial acetic acid and titrate with perchloric acid – acetic acid standard solution. Endpoint is detected by potentiometric titration with glass / reference electrodes.
CH₃COONa + HClO₄ → CH₃COOH + NaClO₄
2. Configuration of instruments and Reagents
| (1) | Instruments | ||
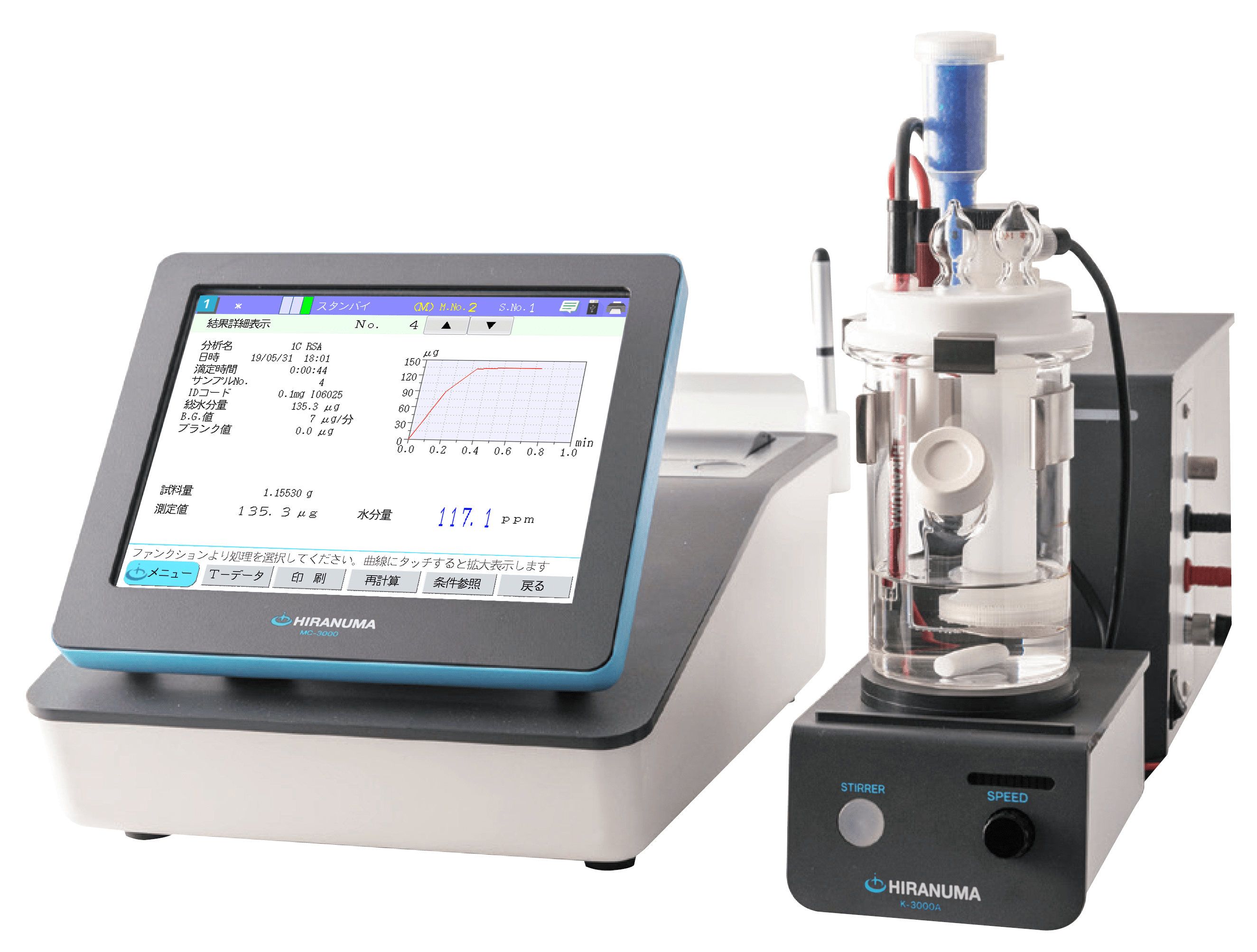
| Main unit | : | Hiranuma Automatic Titrator COM Series | |
| Electrode | : | Glass electrode GE-101B Reference electrode RE-201Z (Inner solution should be changed; it is described below) |
|
| (2) | Reagents | ||
| Titrant | : | 0.1 mol/L perchloric acid in glacial acetic acid standard solution | |
| Titration solvent | : | Glacial acetic acid | |
| Electrolyte | : | Saturated sodium perchlorate in glacial acetic acid (For inner solution of reference electrode) |
3. Measurement procedure
| (1) | Take 0.2 g of the sample into a 100 mL tall-beaker and accurately weigh it. |
| (2) | Add 50 mL of acetic acid and dissolve the sample. |
| (3) | Immerse the electrodes and start titration with 0.1 mol/L perchloric acid in glacial acetic acid standard solution. Perform blank test without sample. |
4. Measurement conditions and results
Examples of titration conditions
Measurement of blank

Measurement of sample

Measurement results
Blank Measurement
| Measurement No. |
Size (g) |
Titrant volume (mL) |
|---|---|---|
| 1 | – | 0.01 |
| 2 | – | 0.01 |
| Statistical result |
Avg. (mL) | 0.01 |
Sample Measurement
| Measurement No. |
Size (g) |
Titrant volume (mL) |
Conc (%) |
|---|---|---|---|
| 1 | 0.2029 | 24.690 | 99.778 |
| 2 | 0.2021 | 24.553 | 99.617 |
| 3 | 0.2010 | 24.440 | 99.701 |
| Statistical result |
Avg. (%) SD (%) RSD (%) |
99.70 0.0805 0.08 |
Examples of measurement curves
|
|
|
5. Note
| (1) | Effect of water on perchloric acid titration Water mixed in sample solution affects to the reaction system of perchloric acid titration because of the leveling effect, which results in a negative effect such as lowered quantitative performance or getting less sensitivity around the end point. Therefore please take care not to mix water in sample solution. Reference electrode for non-aqueous titration should be prepared as described in the following item (2), because water of KCl solution commonly used as inner solution for reference electrode could be mixed in sample solution. |
||||||||
| (2) | Preparation of inner solution for reference electrode The inner solution of the reference electrode RE-201Z is filled with saturated KCl aqueous solution normally. For this measurement, it is necessary to replace inner solution to saturated sodium perchlorate in acetic acid solution. Replacement procedure is described below.
|
||||||||
| (3) | Experiment temperature Acetic acid used as a solvent for the titrant has a relatively large thermal expansion coefficient, and when the temperature changes by 1 °C, the titrant causes a volume change of 0.1 %. For accurate measurement, factor titration and sample measurement should be performed at the same room temperature as much as possible. |
Keywords: Sodium acetate, Perchloric acid titration, Nonaqueous titration, Neutralization titration
*Some measurement would not be possible depending on optional configuration of system.