Description
Specially dried methyl alcohol for use with KF Reagents 1600, 1601 and 1620. For general purpose. Not for aldehydes and ketones. Please note - this item is not returnable.
Specifications
CAS No. 67-56-1
Chem. Form. CH3OHDEA
Controlled: No
Disclaimer: Consult SDS before use. Not for direct food, drug or cosmetic use. For details go to www.gfschemicals.com/terms.asp. For sale by GFS and its authorized dealers only.
Form: Anhydrous
Hazmat: 3
Mol Wt. 32.04
Package Size: 1 L
| Assay 99 % |
|
Water 0.00 - 0.01 % |
Product FAQs
What is Methyl Alcohol?
Methyl Alcohol, also known as methanol or wood alcohol, is a colorless, very volatile liquid with a faint sweet fruity odor similar to that of ethanol. With water, it entirely mixes. Vapors are slightly greater than air in weight and may travel some distance to a source of ignition before returning as flames. If vapors build up in confined places like buildings or sewers, they can explode if set alight. Used to produce chemicals, remove water from automobile and aviation fuels, solvent for paints and plastics, and an ingredient in many consumer products.
Methanol is a one-carbon alcohol that consists of a methyl group and an alcohol group. It serves as an amphiprotic solvent, as a fuel, as a human metabolite, as an Escherichia coli metabolite, as a mouse metabolite, and as a Mycoplasma genitalium metabolite. It's an alkyl alcohol, a one-carbon compound, a volatile organic compound, and a primary drink. It is the conjugate acid of Methoxide.
Volcanic gases, vegetation, and microorganisms all contribute to methanol (ch3oh or ch4o) releases into the environment. Humans may be exposed to methanol through ambient air and during the use of solvents. Blurred vision, a headache, dizziness, and nausea are possible symptoms of acute (short-term) or chronic (long-term) inhalation or ingestion of methanol by humans.
In terms of reproductive, developmental, and carcinogenic effects in humans, there is no information available. The offspring of rats and mice exposed to methyl alcohol by inhalation has been shown to have birth defects. With respect to cancer hazard, methanol has not been classified by EPA.
GFS Chemicals is a leading chemical manufacturer and supplier of Methyl Alcohol in the USA.
What is the chemical formula for Methyl Alcohol?
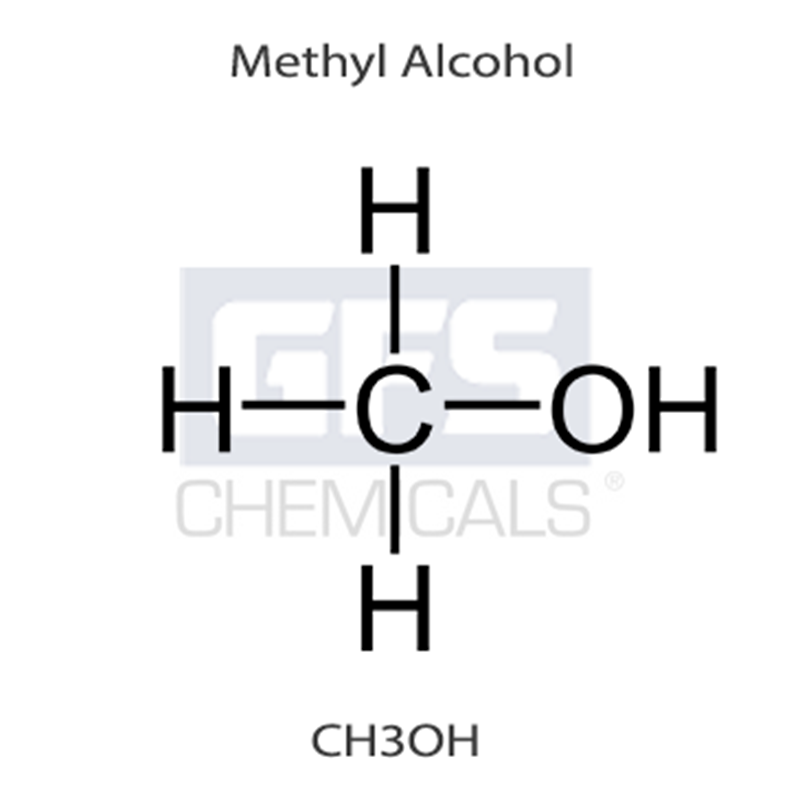
The molecular structure of Methyl Alcohol is CH4O or CH3OH.
What is the molecular weight of Methyl Alcohol?
The molar mass of Methanol is 32.042 g/mol.
What is the melting point of Methyl Alcohol?
The melting point of Methyl Alcohol, also referred to as Methyl Hydrate, is -97.6 °C (-143.68 °F; 175.55 K).
What is the boiling point of Methyl Alcohol?
The boiling point of Methyl Alcohol is 64.7 °C (148.46 °F; 337.85 K).
What is the solubility of Methyl Alcohol?
Methyl Alcohol (methanol) is miscible with ethanol, ether, benzene, most organic solvents and ketones. It is also soluble in acetone and chloroform.