Description
Hitachi branded Hollow Cathode Lamp Cadmium (Cd)
Light Source
The spectral width of an atomic absorption spectrum is very narrow (usually about 0.01 nm). To measure atomic absorption, a light source with narrower spectral width is necessary. The spectral width of the light source of the spectrophotometer is as wide as 1-2 nm.
Therefore, an atomic absorption cannot be measured with a continuum source. In an atomic absorption photometer, a hollow cathode lamp (HCL) is used. The spectral width of an emission line (bright line) of a hollow cathode lamp is even narrower than a line in an atomic absorption spectrum.
Background (BKG) "What is background?"
In a photoabsorption measurement, an analytical line may be darkened due to causes other than absorption by a target metallic element. This darkening is called background. For example, in the case of sodium chloride (NaCl) in sea water, the wavelengths absorbed by Na, cadmium (Cd), and nickel (Ni) are overlapped. For a specimen containing a large quantity (several percent) of NaCl, like sea water, a trace amount of (ppm) Cd or Ni cannot be measured accurately. If the background absorption is not corrected accurately, the measurement result will be ruined.
Background(BKG) Correction Method
Let's see how BKG is corrected, in principle.
However, before that... "How to measure only the background absorption" is the point.
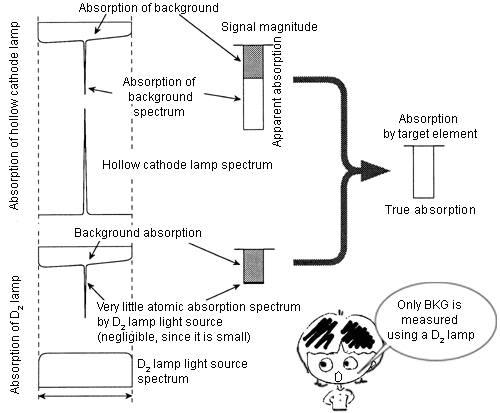
1. D2 (deuterium lamp) correction method
A hollow cathode lamp and a D2 lamp are used as light sources (The following diagram shows the optical system). Please imagine that there are two photometers, one using an HCL as its light source, and one using a D2 lamp as its light source. The signals from the two photometers are electric signals which are processed and distinguished.
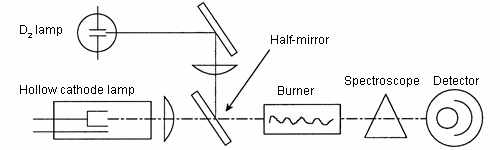
- Photometer using HCL as its light source: Measures atomic vapor absorption + BKG absorption.
- Photometer using D2 lamp as its light source: Measures only BKG absorption. This is the point. When a D2 lamp is used as the light source, the wavelength interval is dependent on the slit width of a spectroscope, which is much larger than the atomic absorption lines (refer to ‘Principle of Atomic Absorption Photometer’). Thus, most of the absorption by atomic vapor is hardly observed (Actually, there is very little absorption).
From (1) - (2), true atomic absorption can be measured.
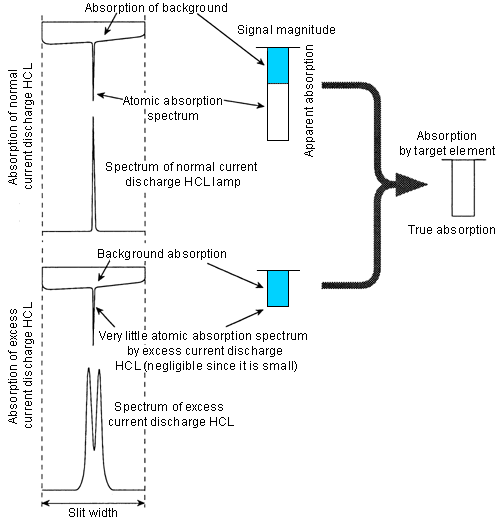
2. Self-absorption correction method
A lighting method for a hollow cathode lamp (HCL) is devised.
- It is normally lit for a certain period. Then, it is lit for a short period with an excess current. This is repeated several tens of times per second.
- Repeatedly, the following spectra can be obtained.
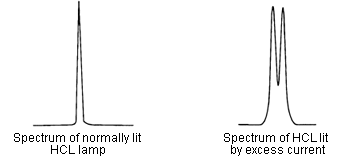
The self-absorption correction method uses the spectrum obtained by lighting with an excessive current instead of the D2 lamp light source spectrum of the previous section.